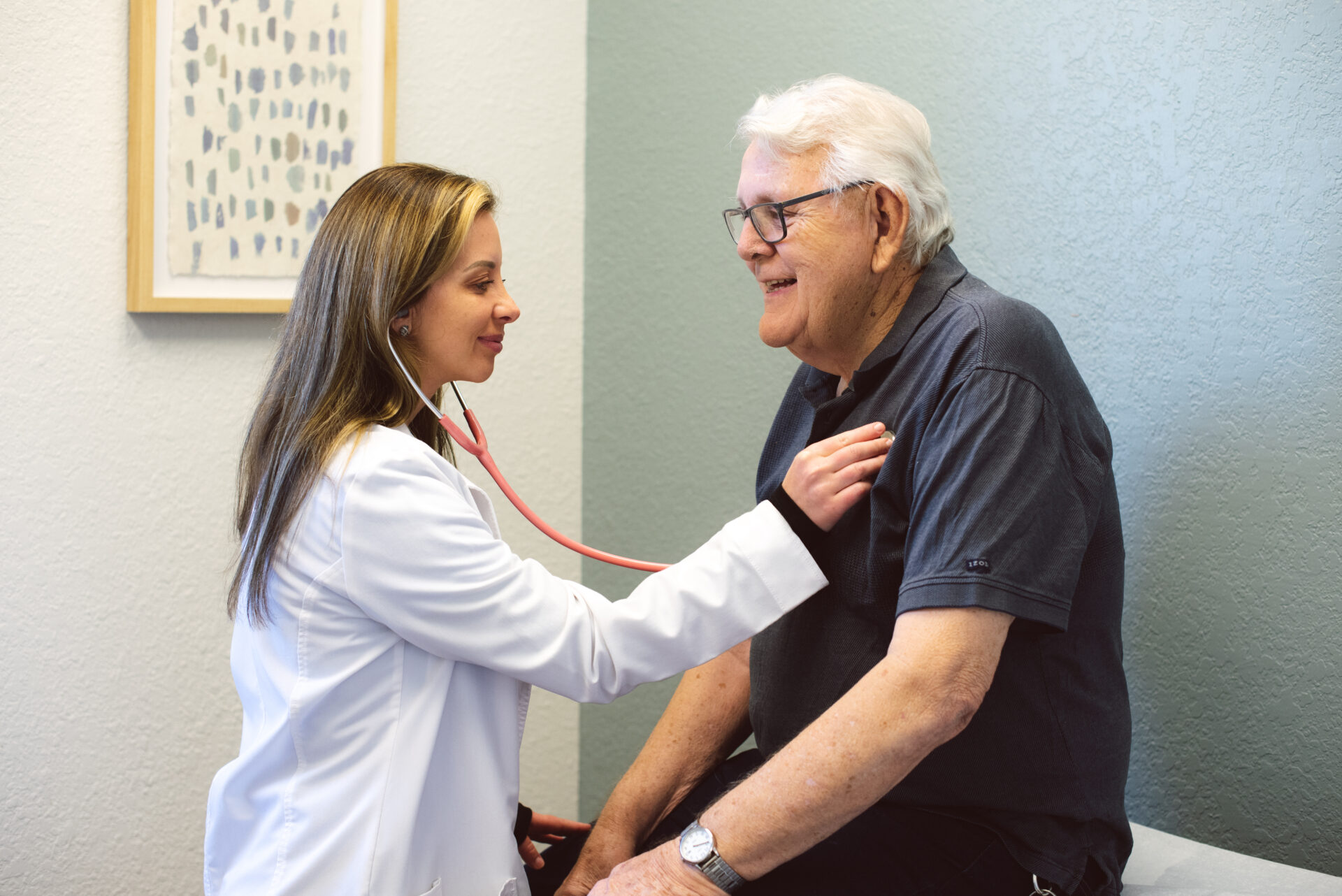
Compassion, Innovation, State-of-the-Art Care
Our Cancer Treatment Options
High-quality, innovative care by the numbers
60+
Clinic
locations
15
Years serving
patients
370K+
Patients
served
Our world-class cancer specialists deliver quality care that is compassionate, easy to access, and personalized to your needs